18 letters
Imagine what your dry AMD patients could really gain

AMD is the leading
cause of legal blindness worldwide
With 90% of cases caused by dry AMD1-5
- Currently there are more than 50 million cases of dry AMD across Europe
- Estimated pooled prevalence is expected to reach 69 million by 2040

EyeMax Mono is specifically designed for dry AMD patients with central vision loss
EyeMax Mono features:
- Foldable, injectable, single-piece intraocular lens (IOL)
- Square-edge design
- Ultraviolet-absorbing, hydrophobic, soft, yellow acrylic
- Design accounts for chromatic and spherical aberration
EyeMax Mono is a clear choice for your patients with advanced dry AMD
EyeMax Mono: unique technology optimises image quality
at the Preferred Retinal Locus (PRL)
- EyeMax Mono is uniquely shaped to deliver an optimised image to all areas of the macula up to 10° from the fovea centre7,8
- A standard monofocal IOL is not designed to do this
- Image quality degrades with increasing eccentricity from the fovea more severely with a standard monofocal IOL than with an ageing crystalline lens9 or EyeMax Mono8
- PRL may also be known as eccentric fixation
To compensate for macular function loss, in some patients fixation may shift to a healthier part of the macula and a PRL may naturally form10
- A PRL may naturally form when fixation shifts to a healthier part of the macula, usually at the border of geographic atrophy (GA)10
The safety profile of EyeMax Mono is consistent with that reported for standard cataract procedures
- Minimal intra-operative complications were reported during EyeMax Mono implantation7,8,11
- Complications resolved with no sequelae and were consistent with those reported for standard monofocal lens implantation7
- To date, few post-operative complications have been reported7,8,11
- Mean post-operative endothelial cell counts were slightly reduced by 7% (244 eyes)7
- Intraocular pressure is reduced or remains stable following implantation7,8
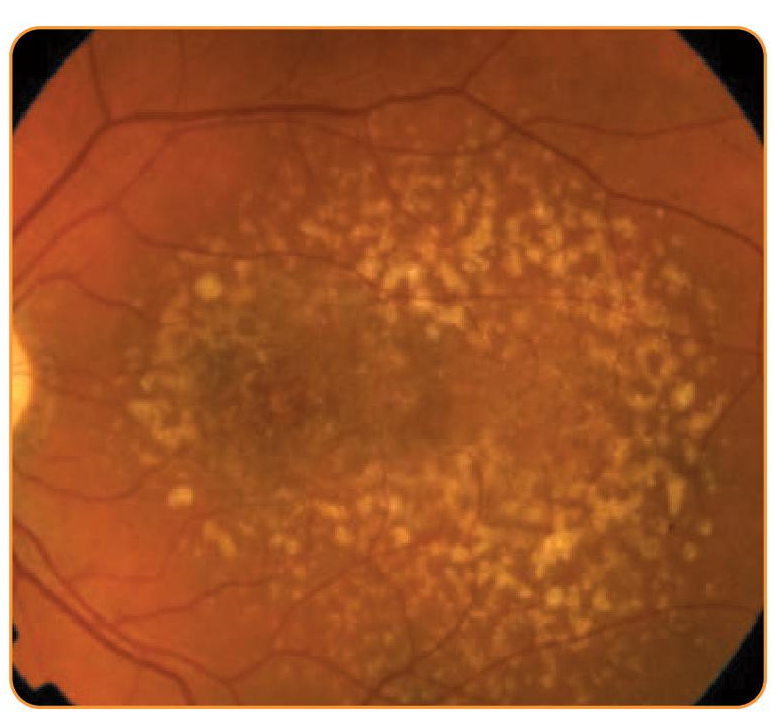
Early/intermediate AMD
EyeMax Mono used as ‘insurance’ against progression to geographic atrophy (GA)
No initial benefit vs standard monofocal IOL; benefit with EyeMax Mono is conferred with the onset of central GA
Caution with rapidly progressing phenotypes (e.g. diffuse trickling)

GA with foveal island
Proceed with caution
No initial benefit over standard monofocal IOL; benefit with EyeMax Mono is conferred when the foveal island is lost
Counsel the patient to expect significant loss of function when the central foveal island is lost
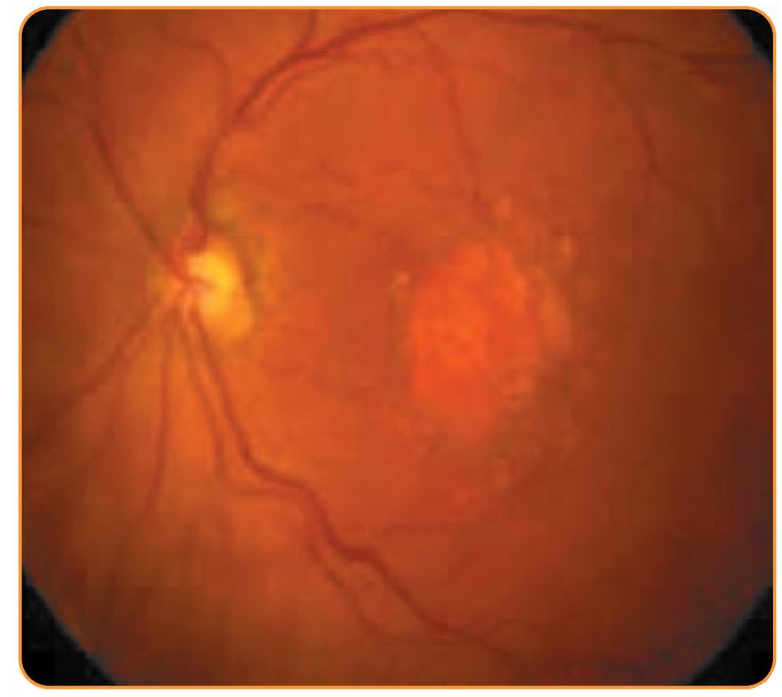
Centre-involving GA with functioning retina within 2 disc diameters of anatomical centre
Ideal initial patient for EyeMax Mono surgery
Ensure glasses are prescribed post-operatively (avoid varifocals)
Allow time for neuroadaptation
Likely no value in using eccentric fixation as criterion for implantation, the patient may adopt this post-operatively even if not present before
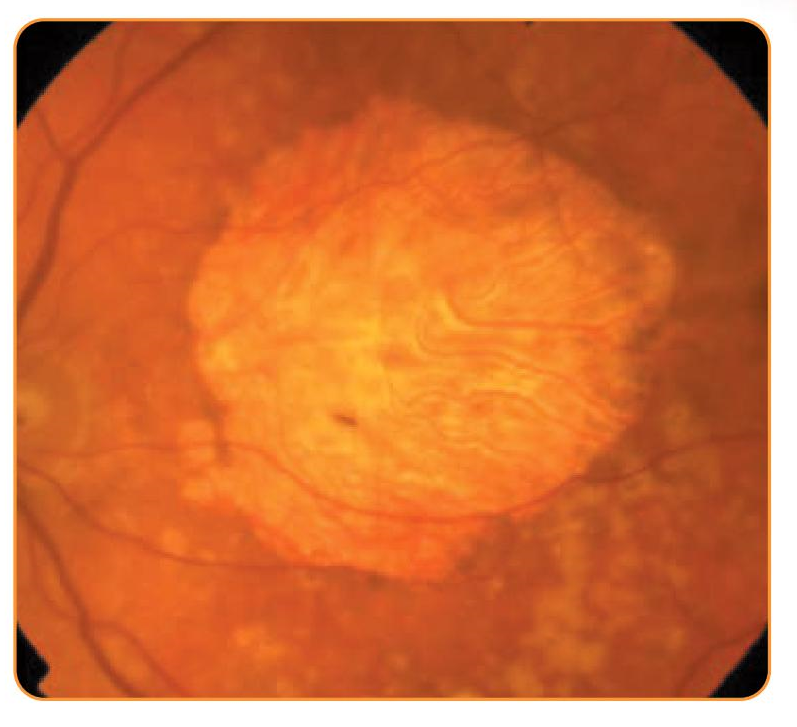
Centre-involving GA with no functioning retina within 2 disc diameters of anatomical centre1,2
Likely no optical benefit of EyeMax Mono vs a standard monofocal IOL
EyeMax Mono should be used in patients with
Cataract requiring surgery
Centre- involving dry AMD
Sufficient remaining macular function within 10° of the foveal centre
References
- Zając-Pytrus HM, et al. Adv Clin Exp Med 2015;24:1099–104.
- Schmidt-Erfurth U, et al. Br J Ophthalmol 2014;98:1144–67.
- Dunbar HM, et al. Ophthalmol Ther 2018;7:33–48.
- Taylor DJ, et al. Ophthalmic Physiol Opt 2018;38:98–105.
- Pennington KL, et al. Eye Vis (Lond) 2016;3:34.
- Wong WL, et al. Lancet Glob Health 2014;2:e106–16.
- Qureshi MA, et al. Eur J Ophthalmol 2018;28(2):198–203.
- Robbie SJ, et al. J Refract Surg 2018;34:718–25.
- Jaeken B, et al. Ophthalmol Vis Sci 2013;54:3594–9.
- Fletcher DC, et al. Ophthalmology 1997;104:632–8.
- Badala F, et al. Poster presented at AAO 2018.
EyeMax Mono surgical procedure
Tips & tricks for surgery
CAUTION
Special consideration should be given to the dimensions of the lens model in relation to the anatomical clearances in the patient’s eye. Long-term clinical outcome must be carefully weighed against the potential benefit associated with the implantation of an intraocular lens. The patient’s clinical progress should be carefully monitored. Patients with any of the following condition may not be suitable candidates for an intraocular lens because the lens may exacerbate an existing condition, may interfere with diagnosis or treatment of a condition, or may pose an unreasonable risk to the patient’s eyesight. Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the benefit/risk ratio before implanting a lens in a patient with one or more of these conditions:
Choroidal hemorrhage, chronic severe uveitis, concomitant severe eye disease, excessive vitreous loss, extremely shallow anterior chamber, medically uncontrolled glaucoma, microphthalmos, non-age-related cataract, posterior capsular rupture (preventing fixation of IOL), proliferative diabetic retinopathy (severe), severe corneal dystrophy, severe optic atrophy, uncontrollable positive pressure and zonular separation (preventing fixation of IOL)
- As with any surgical procedure, there is a risk involved. Potential complications accompanying cataract or implant surgery may include, but are not limited to the following: corneal endothelial damage, infection (endophthalmitis), retinal detachment, vitritis, cystoid macular edema, corneal edema, pupillary block, cyclitic membrane, iris prolapse, hypopyon, and transient or persistent glaucoma.
- The safety and effectiveness of intraocular lens implants have not been substantiated in patients with pre-existing ocular conditions (chronic drug miosis, glaucoma, amblyopia, diabetic retinopathy, previous corneal transplant, previous retinal detachment and/or iritis, etc.). Physicians considering lens implantation in such patients should explore the use of alternative methods of aphakic correction and consider lens implantation only if alternatives are deemed unsatisfactory in meeting the needs of the patient.
- The long-term effects of intraocular lens implantation have not been determined. Therefore, physicians should continue to monitor patients postoperatively on a regular basis.
- Patients with preoperative problems such as corneal endothelial disease, abnormal cornea, macular degeneration, retinal degeneration, glaucoma and chronic drug miosis may not achieve the visual acuity of patients without such problems. The physicians must determine the benefits to be derived from lens implantation when such condition exists.
- Postoperative distension of the capsular bag with variable amounts of anterior chamber shallowing and induced myopia have been associated with capsulorhexis techniques and implantaion of PMMA, silicone and acrylic posterior chamber lenses (Holtz, 1992)
It is recommended that viscoelastic be removed from the eye at the close surgery with emphasis on the space between the posterior capsule lens. This may be accomplished by gently depressing the optic posteriorly with the I/A tip and using standard irrigation I aspiration techniques to remove the viscoelastic agent from the eye. This should force any trapped viscoelastic anteriorly where it can be easily aspirated.
*NOTE: Implantation of intraocular lenses should not be performed in patients under eighteen years of age.
- Do not resterilize these intraocular lenses by any methods.
- Use only sterile intraocular irrigating solutions to rinse and/or soak lenses.
- Handle lenses carefully to avoid damage to lens surfaces or haptics.
- Do not attempt to reshape haptic in any way.
- A high level of surgical skill is required for intraocular lens implantation. The surgeon should have observed and I or assisted innumerous implantations and successfully completed on or more courses on intraocular lens implantation before attempting to implant intraocular lenses.
Do not reuse. Reuse may lead to toxic effect.

